3D Bohr Model Of Atom. An atom model project is a great way to introduce students from elementary school through high school to the 3d concept of the atom, and how the protons and neutrons in the nucleus relate geometrically and in terms of size to the electrons spaced at. The bohr model is integral to our modern understanding of atomic structures.
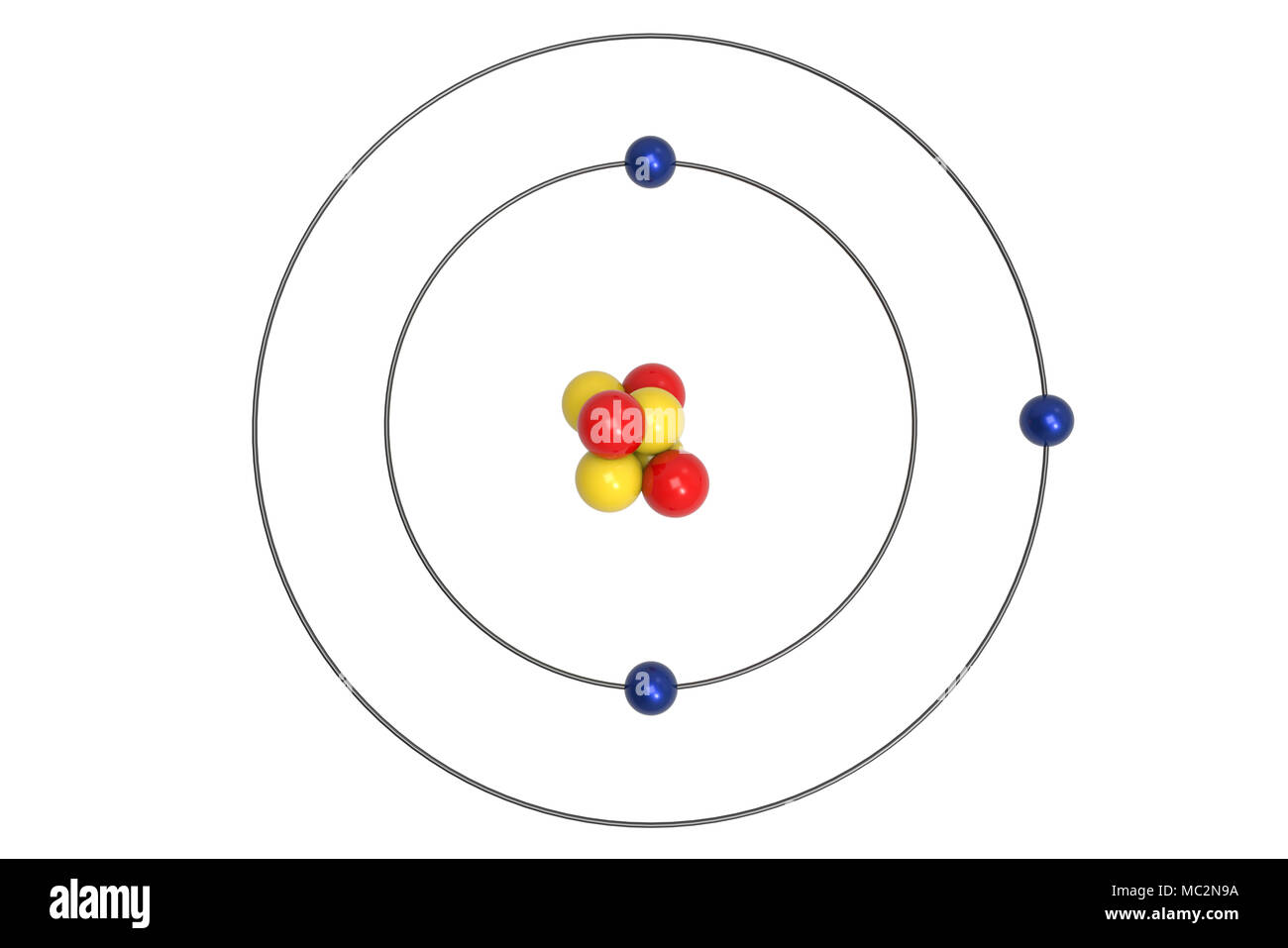
3d models new & unrated price. Sir william ramsay and morris travers discovered the element neon in 1898. Bohr model of lithium (li) 2, 1:
Choose To Visualize The Atom With The Bohr Model, Complete With 3D Electron Shell And Nucleus And Animated Orbiting Electrons, Or The Quantum Mechanical Model, Complete With Animated Electron Cloud.
Unlike earlier models, the bohr model explains the rydberg formula for the spectral emission lines of atomic hydrogen. Bohr model of carbon (c) 2, 4: Planetary model =>erwin schrödinger's model:
Plum Pudding Model =>Ernest Rutherford's Model:
The bohr model of neon is drawn with only two electron shells, the first shell contains 2 electrons and the second shell contains 8 electrons. Bohr’s model was not able to define the effect of magnetic field and electric field on the spectra of atoms. See more ideas about bohr model, atom model, science projects.
This Model Was Proposed By Niels Bohr In 1913.
Bohr model of nickel atom. This blog will include the following components: This drawing represents a bohr diagram of a.
Bohr Model Of Fluorine (F) 2, 7:.
The bohr model of carbon is drawn with only two electron shells, the first shell contains 2 electrons and the second shell contains 4 electrons. How to make a model of the neon atom. Some images may contain licenses that you cannot use for commercial activities.
In The Year 1913, Niels Bohr Proposed An Atomic Structure Model, Describing An Atom As A Small, Positively Charged Nucleus Surrounded By Electrons That Travel In Circular Orbits Around The Positively Charged.
Most students learn about atoms and characteristics of the elements on the periodic table in middle and high school science classes. In this representation, the electrons are rotating in a 2d plane unlike the bohr model which suggests the electrons move in a 3d path around the nucleus. This is a bohr model of the element technetium, tc.
0 Comments